
DeviceTalks Managing Editor Kayleen Brown and Advanced Sterilization Products Global President Chad Rohrer at DeviceTalks Boston 2024. [Photo by Jeff Pinette for DeviceTalks]
What does the future hold for medical device developers?
That’s the question we try to answer at DeviceTalks to help medical device companies design and manufacture more effective products at lower costs, positioning them for quicker clinical adoption.
We invited over a dozen device companies to answer that question and more in our recording studio at DeviceTalks Boston 2024 in May. You can find their answers on our new DeviceTalks Spotlight page — and you can even join the conversation by asking questions of your own directly to our experts.
Here’s a small sampling of some of their insights for anyone designing medical devices.
The need for empathy

Nova Lead Engineer Catherine Jameson [Photo courtesy of Nova]
Catherine Jameson, lead engineer at Nova, hopes medical device designers will work harder to incorporate everyone in design, including patients.
“We talk a lot about empathy, but sometimes that can look like pity or sympathy,” she said.
What device developers really need to do is include patients’ experience in the design process “so we’re respecting what they already know about their bodies and what they need.”
Think beyond the prototype
Diane Hunter, director of engineering at Acme Monaco, said the engineering and manufacturing company anticipates future devices will require more custom designs for unique approaches to solve problems. Device designers should take unorthodox steps with new designs, she said, but the reality of manufacturing devices — like the tolerances of materials — should be given equal weight.
“It’s not that hard to make a prototype of a product,” Hunter said. “But when you want to take that to commercialization and you’re looking at high volumes of manufacturing, you not only want the product to be a great design but you want it to be cost-effective.”
Risk of AI bias
Jeanette Numbers, founder and CEO of Nova, warned the risk of bias in device design could be compounded by the introduction of artificial intelligence if designers aren’t importing data on broad patient populations.
“If we’re not training our AI modules to include everyone, then we’re just going to get more of the same,” she said.
The importance of biocompatibility

PSN Labs VP and Principal Scientist Matthew Heidecker [Photo courtesy of PSN Labs]
Matthew Heidecker, VP and principal scientist at PSN Labs, said regulators who previously focused on the impact of chemicals like cadmium, lead, and mercury are now homing in on organic molecules used in device manufacturing. “The FDA really pushes for manufacturers to understand that your device works from a biocomp standpoint on day one,” Heidecker said. Manufacturers need to know what impact long-time use, reuse, sterilization and other physical demands will have on device materials and in patients.
A fresh look at sterilization
Chad Rohrer, global president of Advanced Sterilization Products (ASP), advised medical device designers to think about how their devices will withstand sterilization “as early as possible.” As ASP and other sterilization leaders look at ethylene oxide (EtO) alternatives and modified approaches in light of health risks and new regulations,
Rohrer said AI could play a role.
“Today, up to 70 percent of instruments that come on a tray are not used … and you’re reprocessing them still,” he said.
Rise of digital modeling
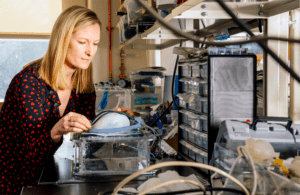
Ellen Roche in the lab with an implantable ventilator prototype [Photo by M. Scott Brauer/MIT]
Massachusetts Institute of Technology biomedical professor Ellen Roche said the use of digital twins like those developed by Dassault Systemes will continue to accelerate device design and development. Used largely in building prototypes, digital simulation will ultimately assist in clinical trials and clinical treatment.
“There are multiple stages at which these tools can benefit the overall workflow,” Roche said.
Adoption challenges include training and infrastructure to run the digital tests. “But we’re working to democratize it in some way and using AI” to accelerate the simulation process, she said.
Easier upgrades

Kevin Dempsey is senior director of business development at Emphysys, a Tecan Group Company. [Photo courtesy of Tecan Group]
Kevin Dempsey, senior director of business development at Emphysys, a Tecan Group Company, said he’d like to see the medical industry adopt the dynamics of consumer technology with multiple updates and enhancements.
“There are many fantastic ideas out there, but we need to bring them to the patient as soon as we can,” Dempsey said.
Medical device developers could build a simple but powerful implantable device that’s designed to accept upgrades through the years so patients benefit from the latest advances, he suggested.
Finding common platforms

KeborMed founder and CEO Dr. Radu Iancu [Photo courtesy of KeborMed]
Dr. Radu Iancu, CEO and founder of KeborMed, envisions the day when medical device manufacturers stop trying to solve connectivity and cybersecurity concerns independent of one another. Instead, the industry will settle on a small number of platforms upon which devices can operate.
“Once people understand that it’s much easier to raise all ships at the same time — and I’m talking about from the largest med device company to the earliest startup — that’s when it’s becoming a tidal wave,” Iancu said.
You can find the entire set of interviews at DeviceTalks.com.





















